By Jeffrey H. Tepper, Geology Department, University of Puget Sound
Introduction
The sediment on the bottom of a lake exerts an important influence on water quality and aquatic life. For example, sediment may function as a sink and/or a source for nutrients, heavy metals, and other pollutants, and it is home to benthic organisms. In addition, lake sediments are an archive of lake history and can record changes in climate, water quality, or land use within the drainage basin.
For the past ten years, students and faculty in the Geology Department at the University of Puget Sound have been studying lakes in the South Puget Sound area, focusing in particular on those in and around the city of Lakewood, Washington. Although these lakes are near one another, they differ significantly in water chemistry and in overall health. A goal of our studies has been to use sediment cores to reconstruct the environmental histories of individual lakes, identify human impacts, and thereby develop a better understanding of the causes of ongoing water quality problems. This article compares two neighboring lakes in Lakewood — Gravelly Lake and Waughop Lake – that share the same geologic origin but have responded very differently to human disturbance over the past 150 years.
Overview of Lakewood area lakes
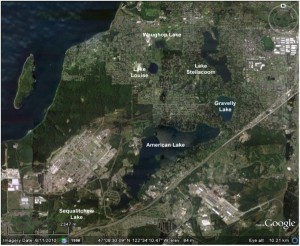
Fig 1. Google Earth image showing lakes in the Lakewood, WA, area. The prominent SW-NE trending road east of American Lake is Interstate 5.
A satellite image of the Lakewood area (Fig. 1) shows half a dozen lakes, the largest of which is American Lake. With the exception of Lake Steilacoom, all of these are natural kettle lakes that occupy depressions formed by the melting of ice blocks during glacial retreat about 13,000 years ago. The area is underlain by a heterogeneous blanket of glacial and non-glacial sediment up to 2000 feet thick, which hosts a shallow unconfined aquifer as well as a deeper “sea level aquifer” (Dinicola, USGS SIR 2005-5035). The highly permeable near-surface sediments facilitate shallow groundwater flow between many of the lakes; for example, allowing water from American Lake to feed springs in Gravelly Lake, which in turn feeds springs in Lake Steilacoom.
Gravelly Lake
Gravelly Lake, with a surface area of 148 acres and maximum depth of about 18 meters, is one of the deeper lakes in the area. It receives surface runoff from a small drainage basin (420 acres) but its main water sources are springs, some fed by shallow interflow from American Lake and others from deeper aquifer(s). Gravelly Lake has no surface outflows. This lake originally attracted our attention because of its unique water chemistry, which is distinct from the adjacent lakes in having higher pH and alkalinity, higher calcium (Ca) and magnesium (Mg), and, most notably, higher silicon (Si), which promotes annual diatom blooms. The water is also unusually clear (with Secchi depths of up to 9 meters) and turns a bright turquoise blue every summer.
University of Puget Sound student Ben Shapiro studied the water chemistry of Gravelly Lake for his thesis in 2009 and was able to show that the main water source for the lake is the “deep groundwater” springs, which are also the source of the Si-rich water. This led to the question of how these springs acquire their Si-rich character. Two hypotheses were proposed: (1) the waters are coming from a deep aquifer where higher temperature and a longer residence time promote greater dissolution of silicate minerals, or (2) the waters are coming from a shallow aquifer that has been contaminated by septic system effluent containing sodium silicate, a component of detergents.
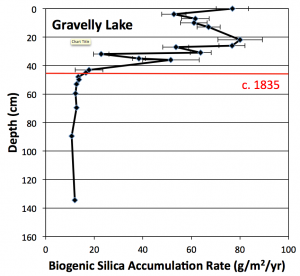
Fig 2. Biogenic silica accumulation rate in Gravelly Lake. The onset of increasing accumulation rates coincides with the introduction of cattle and sheep into the area around 1835. Data from unpublished University of Puget Sound B.S. thesis of Paul Woodward (2011).
To evaluate these hypotheses, a 1.5 meter sediment core was collected from the center of Gravelly Lake in May 2010. The core was sliced into sections one centimeter thick and analyzed for a variety of parameters including organic content, biogenic silica (diatom content), phosphorus, carbon and nitrogen isotope ratios, and heavy metals: lead (Pb), zinc (Zn), copper (Cu), and arsenic (As). The 210Pb method was used to date the core, providing high-resolution age control as well as a record of changing sediment accumulation rates over the past 150 years or so. The results of these analyses revealed a series of abrupt changes beginning at a core depth of about 42 centimeters. Above this point, biogenic silica accumulation rates increased five-fold (Fig. 2), phosphorus accumulation rates tripled, and the rate of sediment accumulation, particularly algal matter, also began rising. These shifts, particularly the increased nutrient loading, are consistent with human disturbance of the lake, but the onset of the change – around 1840 – is far too early to attribute to detergents.
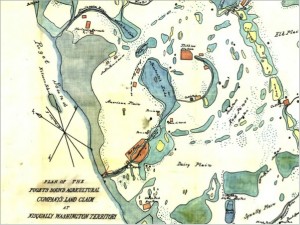
Fig 3. Map of Puget Sound Agricultural Company lands around Gravelly Lake in 1855. Note that north is not at the top on this map. American Lake is the large labeled water body in the center of the map; Gravelly is the smaller labeled lake just north of American Lake. The stippled pattern indicates lands used for pasturing of livestock. (Map downloaded from http://www.sos.wa.gov)
However, the shifts do coincide with an earlier milestone in the European settlement history of the Lakewood area: the establishment of Fort Nisqually by the Hudson Bay Company in 1833. One of the company’s primary business goals was to sell meat to settlers, so within a few years the land around the fort was home to thousands of head of cattle and sheep. An 1855 map (Fig. 3) clearly shows that the area around Gravelly Lake was part of this pasturing operation, and associated animal waste was almost certainly the cause of the increased nutrient loading and the resulting diatom blooms. This scenario also implies that Si-rich water is a long-standing natural trait of Gravelly Lake, but it was only after European settlement that the supply of other nutrients, particularly phosphorus, was high enough to trigger the diatom blooms that are recorded in the upper 40 centimeters or so of the sediment core. Geologic evidence suggests that Gravelly Lake sits above a breach in the confining layer that caps the deeper aquifer, and this may explain why Si-rich waters feed this lake but not others in the area.
Fort Nisqually was closed in 1869 and land use around Gravelly Lake has since transitioned from agricultural to residential. Phosphorus loading has continued to increase, however, probably due at least in part to internal cycling, and diatom blooms occur each summer. These blooms are also responsible for the unusual blue color of the lake in the summer. Photosynthetic CO2 uptake by the diatoms induces precipitation of minute calcite crystals, which when suspended in the water column give it a blue color. In addition, the diatoms help to protect Gravelly Lake from excessive growth of blue-green algae because the diatoms bloom earlier in the spring and deplete the surface waters of phosphorus.
Waughop Lake
Located only a few kilometers northwest of Gravelly Lake, Waughop Lake (Fig. 1) is another former kettle that has been impacted by agricultural activity over the past 150 years. The response of this lake has been quite different, though: Waughop today is a highly eutrophic lake that experiences serious toxic algal blooms and is ranked as the most toxic lake in western Washington. Water clarity is poor, particularly during the summer (with a Secchi depth of less than 1 meter), the surface is often covered with an algal scum, and the lake, located in Fort Steilacoom Park, is commonly closed to all recreational uses during the summer.
Waughop Lake is smaller (33 acres) and shallower (with a maximum depth of about 3 meters) than Gravelly Lake, and is fed by a combination of springs, surface runoff from surrounding athletic fields, and a storm water drain from nearby parking lots; there is no surface outflow. Compared to Gravelly Lake, Waughop Lake waters are dilute and low in silicon.
Beginning around 1870, the land around the lake was part of the grounds of the state mental hospital and was used for dairy and hog farming. Manure and other agricultural wastes were dumped directly into the lake until 1965. The first recorded algal bloom occurred in 1973 and since 2007 the lake has exceeded safe levels of microcystin on 80% of the test dates.
Considering the land use history around Waughop Lake, it seems likely that bottom sediments are a significant source of nutrients that feed the algal blooms. To assess the amount and composition of nutrient-rich bottom sediment, the lake was cored in September 2012 and 6.5 meters of core were recovered. At 4.2 meters depth, the core passed through a volcanic ash layer deposited by the 7700 BP eruption that produced Crater Lake. Given that another 2.3 meters of lake sediment lie beneath this ash, it seems likely that our Waughop Lake core preserves a record extending back to the time of glacial retreat roughly 13,000 years ago.
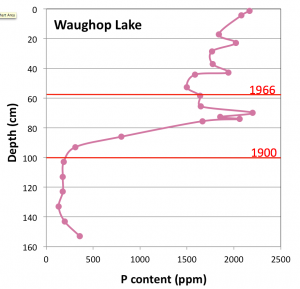
Fig 4. Phosphorus content of Waughop Lake sediment. The dramatic increase after 1900 coincides with use of the lake as a disposal pond for agricultural wastes, a practice that ended in 1965. Dates are based on 210Pb dating of the core. Data from unpublished University of Puget Sound B.S. thesis of Elli McKinley (2013).
Chemical analyses and 210Pb dating of the core show that phosphorus concentrations were low over most of the lake’s history, but increased almost tenfold beginning around 1900 (Fig. 4). At this same time there was an abrupt shift to higher nitrogen isotopic ratios, which are indicative of animal manure and point to agricultural waste as the source of the nutrients. As at Gravelly Lake, this increase in nutrient supply triggered an increase in algal production (revealed by a drop in C/N values) and an increase in sedimentation rates. However, at smaller and shallower Waughop Lake, the effect on biological productivity has been far more dramatic and detrimental.
The problem is further exacerbated by the naturally lower silicon content of the water, which limits the uptake of phosphorus from the water column by diatoms and thus allows greater growth of blue-green algae. Since 1900, the sediment accumulation rate at Waughop Lake has risen from 2000 to 6000 g/m2-yr, a rate that today is more than 20 times higher than that of Gravelly Lake (about 250 g/m2-yr) and almost 50 times higher than that of Lake Louise, the neighboring lake (Fig. 1) that has experienced the least anthropogenic nutrient loading. As a result of its eutrophic conditions, Waughop Lake has become about a meter shallower over the past century and suffers from anoxic conditions that promote toxic algal blooms. Over the next century or two, if nothing is done to address the nutrient loading problem, the lake will likely become a wetland.
Fortunately, efforts have begun to restore Waughop Lake to a healthier condition. Among the necessary first steps will be better quantification of the various nutrient sources that are feeding the algal blooms and an assessment of the options for reducing the annual cycling of phosphorus from the sediment to the water column. Waughop Lake probably never was and never will be as clear as its deep, silicon-rich neighbor, Gravelly Lake, but we hope that a combination of science, funding, and community support can return it to a picturesque and healthy state.









