by Alexander D Reyes, Ph.D. candidate, Washington State University
Co-authors: Barry C. Moore, Timothy N. Taylor, Bryan Jones.
(Alexander Reyes is the winner of WALPA’s 2021 Nancy Weller Memorial Scholarship for PhD graduate students.)
Figure 1. Topographic setting for Omak Lake, Washington. Inset: Washington state map showing Omak Lake (red dot).
Alkaline lakes are often found in graben and fault basins and in sites of active or recent volcanism where surface water outflows are absent or greatly reduced compared to inlet volumes (Stüekena et al. 2015). Omak Lake is located in Okanogan County in north central Washington, within the western portion of the Reservation of Colville Confederated Tribes (CCT). It is the largest saline lake in the state (Bennet 1962 and WSU 1978), with a lake surface area of 3,820 acres (~1,550 ha). The surface elevation is approximately 935 ft (285 m) above sea level. The maximum lake length is 7.5 miles (12 km), and average width is 0.75 mile (1.2 km). Estimated average lake volume is 30,710,000,000 ft3 (705,000 acre-ft, 869,610,360 m3), average depth is 185 ft (56 m) and maximum depth is ~330 ft (100 m).
Generally, saline lakes have less biodiversity than near-neutral pH lakes due to the limited number of taxa that can survive saline conditions. However, significant biota have adapted to higher pH and salinity conditions and, practically due to lack of competition, can thoroughly exploit the material and energy resources of these specialized niches, so that saline or alkaline lakes are often among the most productive ecosystems on the planet (Wetzel 2001; Stüeken et al. 2015). Omak Lake waters are alkaline. They contain high concentrations of carbonate and had an average pH of about 9.0 from 2019 through 2021.
The native Omak Lake fish community includes peamouth (Mylocheilus caurinus), bridgelip sucker (Catostomus columbianus), redside shiners (Richardsonius balteatus), and sculpins (Cottus sp.) (Kucera et al. 1985). Lahontan cutthroat trout (Salmo clarki henshawi) (LCT) were introduced to Omak Lake in 1968 to create a sport fishery; they are an example of a fish species that has adapted to high salinity environments (Kucera et al. 1985). Most natural populations of LCT are listed in the Endangered Species Act of 1975 (National Fish and Wildlife Fundation 2020). The original LCT stocked in Omak Lake in 1968 were taken from Summit Lake in Nevada. In 1969, subsequent egg-harvesting from Omak Lake resulting from the mixed cross of Summit/Heenan Lake stocks produced a genetically stable strain (Eddy et al. 2020).
Project goals
Overall, this project’s goals are to assess the Omak Lake LCT population’s seasonal dietary trends and age structure. The implementation of stomach content analysis (SCA) in conjunction with stable isotopes ratios will be the tools to propose a theoretical food web for Omak Lake that sustains LCT.
Methods
LCT were collected in littoral areas of Omak Lake using fyke nets in the spring (May) and gillnets in the summer (August) and fall (October) during 2018 (Spring only), 2019, 2020 and 2021. Collected fish were separated and measured in the field to the nearest millimeter of total length, and sorted into length categories (150-249, 250-349, 350-449, 450-549, 550-649, and >650mm). Otoliths were extracted to determine age and stomachs were removed for stomach content analysis (SCA). Whole stomachs were detached from the fish gut and placed in labeled Ziploc® bags with 70% ethanol preservative (Bowen, 1996).
SCA involved removing all identifiable prey species from the stomachs. Undigested prey items (over 85% of stomach items) were counted and identified at least to order, with some fresh prey items identified to species. Individuals from each order were measured to the nearest millimeter; lengths were used to estimate wet weights from length/weight regressions obtained from the literature. Empty stomachs were excluded from the analyses. Total weight of each type of prey in the stomachs was estimated by summing the total weights for all individuals (Skinner et al. 2014).
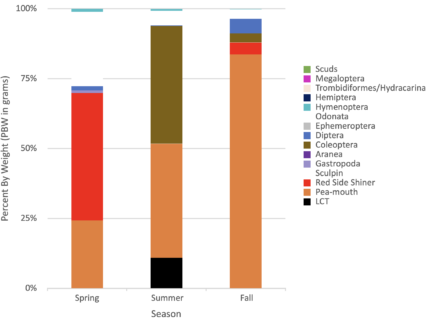
Stable isotope analyses (SIA) were performed on LCT liver tissues. Previous research on other CCT lakes has shown that liver tissues provide representative results for estimating prey contributions to fish diets (Skinner et al. 2016, 2017). Benthic/littoral invertebrates are important prey components in Omak Lake LCT diets. LCT prey items for SIA included macroinvertebrates, zooplankton, and fish (sculpins, redside shiners, suckers, and peamouth) and were collected at approximately the same times as LCT collections (more or less once/week).
Macroinvertebrates were collected in littoral areas with a D-framed kick-net. Sagittal otoliths were collected from all fish for ageing using methods described by Schneidervin and Hubert (1986). A complete water quality assessment of the lake (physical parameters, macrofauna, and nutrients) complemented the diet analysis for LCT in Omak Lake.
Results & Discussion
Interannual diet:
Contrary to 2019 data, there was an increase in piscivory by LCT during 2020 and 2021. That is good to ensure the proper growing and sustainability of the Omak’s sport fishery. Higher caloric intakes for trout minimize the energetic cost of preying in conjunction with reduction of cannibalism of the hatcheries released in spring. The amount of biomass was consistent for 2019 and 2020 (776.3g in 2019 and 775.7g in 2020), a bit different for 2021 perhaps due to a higher number of stomachs sampled (1,753.41g). However, invertebrates in 2019 constituted almost 36% and 32% for 2021 of the total prey items identified in stomachs but less than 20% in 2020.
Seasonal diet:
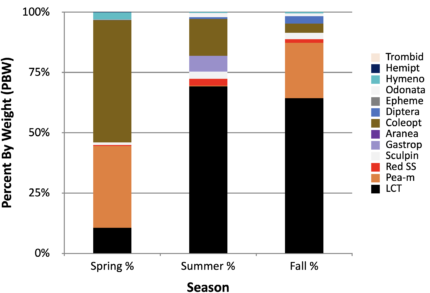
Surprisingly, the seasonality of terrestrial bugs (beetles) only changed in 2020. In 2018, 2019, and 2021, terrestrial beetles, ants, bees, and other insects comprised most of the diet items found in LCT stomachs across all spring and part of the summer. Thus, 2020 was the year with highest piscivore feeding and the only time when terrestrial beetles were nearly absent in spring until mid-summer when they comprised 30% of diet.
Spatial/ontogenetic diet:
LCT showed shifts in diet over their life cycle. The smallest LCT (1-2 years-old) ate solely invertebrates, with fish encompassing a larger percent of the diet as fish grew. There were seasonal trends in diet as well. Terrestrial prey is abundant during spring and probably early summer. By late summer and early fall, more fish are eaten. Perhaps the seasonal shift to prey on invertebrates occurs because of a shift in food availability. Mid-sized (300 mm to 550mm) growing LCT preyed on Odonata (dragonfly, damselfly), Hemiptera (water boatman), and Hymenoptera (terrestrial ants, wasps, bumblebees). Inputs of invertebrates by air currents are well received by LCT, who will opportunistically eat floating invertebrates on the water surface. Larger and older fish (>550mm, >5-years) relied sometimes on insects but mostly large fish prey (suckers or large peamouth, with cannibalism of small LCT observed in 2019). Large fish specimens are scarce and difficult to collect. Some stomachs sampled were empty and few collections occurred either in spring or summer, none in fall. The lack of large LCT is perhaps due to lack of large fish available for prey.
In addition, 2021 results suggest that the fire in September 2020 on the west slopes of the lake watershed may not have reduced the potential supply of terrestrial invertebrates. Perhaps the major portion of terrestrial inputs occur in the eastern end of the lake.
Finally, Dipterans, mostly chironomids (blood worms, midges, flies, mosquitoes, blackflies, etc.) were also significant LCT prey items in all seasons for 2018-2021. They were present in the lake during all sampling events/season and were sometimes abundant in LCT stomachs. Perhaps dipterans are even more important than biomass percentages suggest, especially during times of low prey fish accessibility.
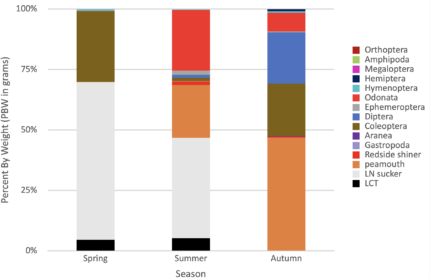
Figure 4. Stomach content analysis for 2021. Beetles and other terrestrial inputs significant in diet.
The isotopic value of nitrogen-15 (δ15N) helps to assign a position in food web ecology. A combination of stable isotopic analysis (SIA) and stomach content analysis (SCA) improves the reliability of ecological data gathered from lakes (Michael H. Meeuwig, 2017). The knowledge of food web ecology is key to developing a sustainable sport fishery. LCT was confirmed as the top predator and constitute the only game fish in Omak Lake. The isotopic nitrogen-15 signal for an adult LCT had an average value near 15‰, that is expected due to the piscivore nature of an adult LCT. The baseline of the foodweb is still unclear, Diptera and Amphipoda had the lowest values of nitrogen-15.
In the final phase of my project, both 13C and 15N isotope ratios will provide the tools to propose a food web for Omak Lake. The combination of 3.3 years of SCA data with SIA will certainly increase the resolution of the results and improve the accuracy of the proposed food web. At this time, that is still a work in progress.
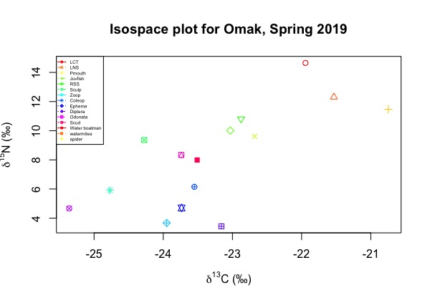
Figure 5. Iso-space plot for spring 2019. Data confirmed LCT (red circle) as top predator in Omak Lake.
I would also like to express my deepest gratitude to the Washington State Lake Protection Association, the Colville Confederated Tribes Fish and Wildlife office (Bryan Jones) and the Water Quality Lab (Barry Moore) of the School of the Environment at WSU Pullman for their contributions and funding of this research.
Works Cited
Bennet, William Alfred Glenn. 1962. Saline Lake Deposits in Washington. Bulletin No. 49, Olympia, WA: State Print Plant.
Bowen, S. H. (1996). Quantitative Description of the Diet (2nd ed.). (B. M. Willis., Ed.) Bethesda, Maryland, USA: American Fisheries Society/Fisheries Techniques.
Michael H. Meeuwig, M. M. (2017, December 17). Food Web Interactions Associated With a Lahontan Cutthroat Trout Reintroduction Effort in an Alpine Lake. Journal of Fish and Wildlife Management, 8(2), 449-464.
National Fish and Wildlife Foundation . (2020). Programs: Lahontan Cutthroat trout. Retrieved October 18, 2020, from https://www.nfwf.org/programs/lahontan-cutthroat-trout#:~:text=Today%2C%20the%20Lahontan%20cutthroat%20is,of%20its%20historic%20lake%20habitat.
Skinner, M.M., B.C. Moore, and M.E. Swanson. 2014. “Hypolimnetic oxygenation in Twin Lakes, WA. Part II: Feeding ecology of a mixed cold- and warmwater fish community.” Lake and Reservoir Management 30 (3): 240-249.
Skinner M.M., A.A. Martin, and B.C. Moore. 2016. Is lipid correction necessary in the stable isotope analysis of fish tissues? Rapid Communications in Mass Spectroscopy 30:881-889.
Skinner, M.M., B.K. Cross, and B.C. Moore. 2017. “Estimating in situ isotopic turnover in Rainbow trout (Oncorhyncus mykiss) muscle and liver tissue.” Journal of Freshwater Ecology 32 (1): 209-217.
Stüeken, E.E, R. Buick, and A.J. Schauer. “Nitrogen Isotope Evidence for Alkaline Lakes on Late Archean Continents.” Earth and Planetary Science Letters 411 (2015): 1–10. https://doi.org/10.1016/j.epsl.2014.11.037
Wetzel, Robert G. 2001. Limnology: Lake and River Ecosystems. 3rd. San Diego, CA, Academic Press.
WSU. 1978. Omak lake database. 4 1. Accessed 03 11, 2019. https://old-www.wsu.edu/cctfish/omak.html