by Michael Lawlor, M.S. candidate, Western Washington University
Algae play a vital role in maintaining the health of freshwater and marine ecosystems. These photosynthetic organisms sequester carbon, are the base of the aquatic food web, and contribute to nutrient cycling. Algae are incredibly diverse organisms that have evolved to inhabit a large range of aquatic conditions (Hemsley 2004). Each taxon has specific physiological requirements and varies in its response to physical and chemical parameters (Wetzel 2001). These specialized requirements allow algae to act as bioindicators of their environmental conditions (Bellinger 2010).
Bioindicators are species, or taxonomic groups, that provide information about ecosystem conditions by their presence, absence, or relative abundance. Phytoplankton were first used as bioindicators of water quality over a century ago (Kolkwitz and Marsson 1908) and are now used globally as a water quality monitoring tool (Chorus 2012). Despite the fact that algae are used as bioindicators around the world, however, the United States, including Washington state, relies primarily on macroinvertebrates as bioindicators of water quality.
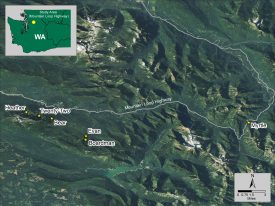
My study examines the algal species composition and water quality of six mountain lakes located in Washington’s Mt. Baker-Snoqualmie National Forest along the Mountain Loop Highway between Granite Falls and Darrington. The study lakes included: Bear, Boardman, Evan, Heather, Myrtle, and Twenty-Two. The lakes are at elevations from 1,750 to 3,200 feet, while their surface areas range from 3 to 45 acres. The lakes are a subset from a previous study (Pfannenstein 2016); my study aims to take a more comprehensive look at the algal species composition and water quality throughout the primary algal growing season.
The primary algal growing season for these lakes typically occurs between June and October when they are free of ice. High elevation lakes experience an annual succession of algal diversity, biomass, and distribution throughout the growing season. Factors controlling succession include temperature, light, nutrient availability, and mortality from both grazing and parasitism (Wetzel 2001). My study was able to capture this succession by sampling multiple times throughout the primary growing season.
My specific objectives were to examine the water quality and algal species composition of the six study lakes, explore how the water quality and algal species composition change throughout their primary growing season, identify algal taxa as bioindicators of specific water quality conditions, and then generate an index to predict water quality conditions based on the algal bioindicators.
I sampled each lake seven times between June and November 2017. At each lake, water quality parameters were measured, and an algae sample collected. All water quality samples were collected at a depth of 0.3 meters to represent surface water conditions. Water temperature, dissolved oxygen, pH, and conductivity were measured in situ using field meters. Additional water samples were brought back for analysis at the Institute for Watershed Studies’ lab at Western Washington University. The lab analyzed: alkalinity, total nitrogen, nitrate, total phosphorus, soluble reactive phosphorus, total and dissolved organic carbon, dissolved silica, chloride, fluoride, sulfate, and heavy metals.
Algae samples comprised planktonic and shoreline algae. Planktonic algae were collected with a 20 µm plankton tow net cast from shore, while shoreline samples were collected by hand from all accessible habitat including woody debris, aquatic vegetation, rocks, and other detritus. These samples were combined into one composite sample for each lake and identified to the lowest practical taxonomic level using light and scanning electron microscopy.
The results of the water quality analysis showed that all six lakes were nutrient poor, slightly acidic (5.4-6.5 pH), and well oxygenated (7-10 mg/L). Nitrate concentrations were all below 0.1 mg/L and all soluble reactive phosphate concentrations were less than 6.0 g/L. Carlson’s trophic state index (1977) suggests that the study lakes ranged from oligotrophic to mesotrophic based on their chlorophyll-a and total phosphorus values. Water temperatures followed the expected seasonal trend of warming from ice-out through mid-summer (August), followed by cooling during September and October before freezing.
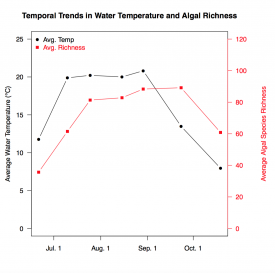
Figure 1: Comparing the changes in mean water temperature and algal species richness throughout the primary algal growing season (n = 6)
Over 340 non-diatom algal taxa were identified in the six lakes. Algal richness was closely linked to water temperature, increasing as the lakes warmed and then decreasing as they cooled, as seen in Figure 1. Out of the 342 taxa identified, only 31 were found in all six lakes, while 133 taxa were unique to a single lake. These results illustrate how unique algae are to their water quality conditions.
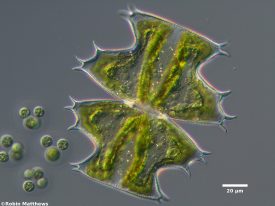
Desmids, an order of green algae, were the most abundant taxonomic group. They are commonly used as bioindicators in other parts of the world, including Europe and Thailand (Coesel 2007; Ngearnpat 2007). Desmids are typically residents of nutrient poor and clear waters that have a neutral or slightly acidic pH (Coesel 2007; Griffiths 1928; Ngearnpat 2007), as found in my study lakes. They are particularly suitable as indicators of good water quality in mountain lakes and ponds. Compared to other taxa, desmids are not readily transported from one lake to another. Therefore, the presence of sensitive desmids usually indicates healthy water quality with little pollution. Moving forward, desmids show promise as bioindicators of water quality in Washington’s North Cascades.
The seasonal changes in algal species richness were quite drastic. Heather Lake’s species richness increased nearly tenfold from the early to the mid-summer samples (August through mid-September) before decreasing significantly towards the end of September. This pattern was repeated in the other lakes and is arguably the most important finding of my study thus far. As there is not always ample funding for intensive lake monitoring projects, we find ourselves relying on smaller sample sizes for our analyses, so we want to make them count. Identifying when the lakes have the greatest algal richness allows us to better characterize their species composition with fewer samples, which reduces the time and funding needed to sample a single lake with accuracy.
The next phase of my project will be analyzing the algal species composition data to identify taxa or groups of taxa that can serve as bioindicators of the water quality conditions found in the study lakes. These indicators will then be applied to a larger group of lakes monitored by the Small Lakes Program at Western Washington University’s Institute for Watershed Studies. This will help determine whether the indicators can be applied at a larger, regional scale.
I would like to thank the Washington State Lake Protection Association, and both the Institute for Watershed Studies and Huxley College of the Environment at Western Washington University for their contributions to my work. Without their funding and support, this research would not have been possible.
Literature cited:
Bellinger, E. G. and D. C. Sigee. 2010. Freshwater Algae: Identification and Use as Bioindicators. John Wiley & Sons, Ltd, Chichester, UK.
Carlson, R.E. 1977. A trophic state index for lakes. Limnology and Oceanography. 22:361-369.
Chorus, I. 2012. Current approaches to cyanotoxin risk assessment, risk management and
regulation in different countries. Umweltbundesamt. Dessau-Roßlau, Germany.
Coesel, P. F., and K. J. Meesters. 2007. Desmids of the Lowlands: Mesotaeniaceae and Desmidiaceae of the European Lowlands. KNNV Publishing. Zeist, the Netherlands.
Griffiths B. M. 1928. On desmid plankton. New Phytologist 27(2):98-107.
Hemsley, A. R., and I. Poole. 2004. The Evolution of Plant Physiology. Academic Press, London.
Ngearnpat, N., Y. Peerapornpisal. 2007. Application of desmid diversity in assessing the water quality of 12 freshwater resources in Thailand. Journal of Applied Phycology 19:667-674.
Pfannenstein, K. 2016. Water quality and algal diversity of ten lakes along the Mountain Loop Highway, Washington. M.S. Thesis, Western Washington University, Bellingham.
Kolkwitz, R. and M. Marsson. 1908. Okologie der pflanzlichen Saprobien. Berichte der
Deutschen Botanischen Gesellschaft 26A:505-19.
Wetzel, R. G. 2001. Limnology: Lake and River Ecosystems. Third Edition. Academic Press, San Diego.