By Samantha Fung (PhD candidate, University of Washington Civil and Environmental Engineering Department.)
Both physical and biogeochemical mechanisms impact the movement of gases, nutrients, and toxins throughout lakes. Unfortunately, more often than not, biogeochemical and physical processes in lakes are studied in isolation, rather than in tandem. Our team at the University of Washington (UW) is studying arsenic-contaminated lakes in the south-central Puget Sound region and working towards bridging this research gap by examining how physical and biogeochemical mechanisms interact to influence the cycling of arsenic in lakes.

Tubing attached to ISCO samplers and placed in Lake Killarney is used to sample lake water for arsenic for current analysis.
Initial project findings demonstrated that lake depth and stratification regime are key in predicting whether arsenic will get transported from bottom sediments into lake waters, where it can be taken up by primary producers (Barrett et al., 2018). Specifically, Barrett et al. (2018; 2019) found that surface water arsenic concentrations were elevated in the study’s shallow, polymictic lake, but not in the study’s deep, seasonally stratified lake. Follow-up work on our study’s most contaminated shallow lake, Lake Killarney (Federal Way, WA), showed that stratification and mixing patterns along with sediment temperatures controlled seasonal patterns in arsenic (Fung et al., 2022).
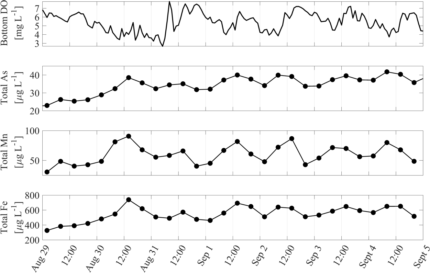
Figure 1: Weeklong timeseries of (a) bottom water dissolved oxygen (DO) and bottom water total metal concentrations: (b) arsenic (As), (c) manganese (Mn), and (d) iron (Fe). For both DO and metals, bottom water measurements were taken at 0.4 m above the lakebed.
We observed that the frequency of lake mixing can vary from monthly to daily in Lake Killarney (Fung et al., 2022); to investigate whether high-frequency mixing affects arsenic concentrations on a daily timescale, we conducted an intensive field study during the summer of 2019. During this study, we collected bottom water samples for arsenic analysis every six hours with an ISCO autosampler along with high-frequency temperature profiles, surface and bottom water dissolved oxygen measurements, and vertical turbulence measurements. Surprisingly, we observed repeated daily cycles in bottom water total arsenic concentration; maximum arsenic concentrations generally occurred in the late morning and minimum concentrations occurred in the evening (Fig. 1b, August 31 – September 5).
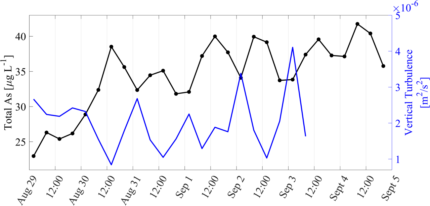
Figure 2: Left axis, black line: residual arsenic concentrations from calculated daily mean. Right axis, blue line: vertical turbulent intensity averaged over the lake depth.
Although 24-hour arsenic cycles are widely documented in rivers and streams (Fuller and Davis, 1989; Nimick et al., 2003; Gammons et al., 2007), there are few observations of this phenomenon occurring in lakes. Further, the timing of the daily minimum and maximum arsenic concentrations observed in lakes is opposite to the timing of the cycle observed in rivers and streams and there is not yet an accepted explanation for what mechanisms may control daily arsenic cycling in lakes. Although this work is ongoing, initial data analysis, which was funded in part by WALPA’s 2022 Nancy Weller scholarship, has suggested that it is likely a combination of biogeochemical and physical mechanisms that create the oscillations in bottom water arsenic in Lake Killarney. We observe that patterns in iron and manganese concentrations mimic the patterns in arsenic concentrations (Fig. 1) and we hypothesize that daily cycles in redox conditions, as indicated by dissolved oxygen levels, may increase and decrease mobilization rates of these metals from solid form into dissolved form at the sediment water interface. Further, we observe that there are peaks in the vertical turbulence, or mixing, that occur before daily increases in arsenic (Fig. 2).
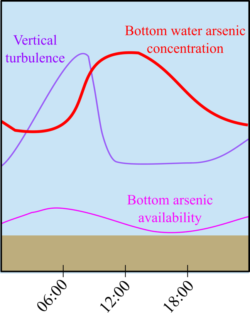
Figure 3: Schematic showing an example of cycles in bottom arsenic availability, vertical turbulence, and bottom water arsenic concentration during one day.
This timing suggests that daily spikes in turbulence may transport dissolved arsenic, iron, and manganese from the near bed region into the lake bottom water and that the combination of the daily redox cycles and mixing processes may lead to the observed 24-hour signals in lake water arsenic (Fig. 3). This research indicates that there is short-term variation of heavy metal concentrations in shallow lakes and that daily cycling should be considered when designing sampling methods to accurately assess environmental health and water quality.
Photos by Dennis Wise and Olivia Hagen/University of Washington
References:
Barrett, P. M., Hull, E. A., King, C. E., Burkart, K., Ott, K. A., Ryan, J. N., Gawel, J. E., & Neumann, R. B. (2018). Increased exposure of plankton to arsenic in contaminated weakly-stratified lakes. Science of the Total Environment, 625, 1606–1614. https://doi.org/10.1016/j.scitotenv.2017.12.336
Barrett, P. M., Hull, E. A., Burkart, K., Hargrave, O., McLean, J., Taylor, V. F., Jackson, B. P., Gawel, J. E., & Neumann, R. B. (2019). Contrasting arsenic cycling in strongly and weakly stratified contaminated lakes: Evidence for temperature control on sediment–water arsenic fluxes. Limnology and Oceanography, 64(3), 1333–1346. https://doi.org/10.1002/lno.11119
Fuller, C. C., & Davis, J. A. (1989). Influence of coupling of sorption and photosynthetic processes on trace element cycles in natural waters.
Fung, S. R., Hull, E. A., Burkart, K., Gawel, J. E., Horner‐Devine, A. R., & Neumann, R. B. (2022). Seasonal patterns of mixing and arsenic distribution in a shallow urban lake. Water Resources Research, 58(10). https://doi.org/10.1029/2022wr032564
Gammons, C. H., Grant, T. M., Nimick, D. A., Parker, S. R., & DeGrandpre, M. D. (2007). Diel changes in water chemistry in an arsenic-rich stream and treatment-pond system. Science of the Total Environment, 384(1–3), 433–451. https://doi.org/10.1016/j.scitotenv.2007.06.029
Nimick, D. A., Gammons, C. H., Cleasby, T. E., Madison, J. P., Skaar, D., & Brick, C. M. (2003). Diel cycles in dissolved metal concentrations in streams: Occurrence and possible causes. Water Resources Research, 39(9). https://doi.org/10.1029/2002WR001571











